Biotech Incubator and Services
We identify new, early-stage biotechnology companies to join the LIMR incubator, and once on board, offer LIMR's ACAPRENEURIALTM approach and services to help them launch and succeed.
Why LIMR to advance your research? Read more about LIMR's available equipment, cores and services.
Download the Research Facilities Booklet (PDF)
Costs – A common rent schedule is available for for-profit and non-profit tenant clients. Space plus overhead costs currently are approximately $65 per sq. ft., plus additional ad hoc services costs as contracted. Companies may offer equity in trade for space and services, at LIMR's discretion.
For more information, contact Matt Finley, Director of Research Administration, at 484.476.8173 or [email protected].
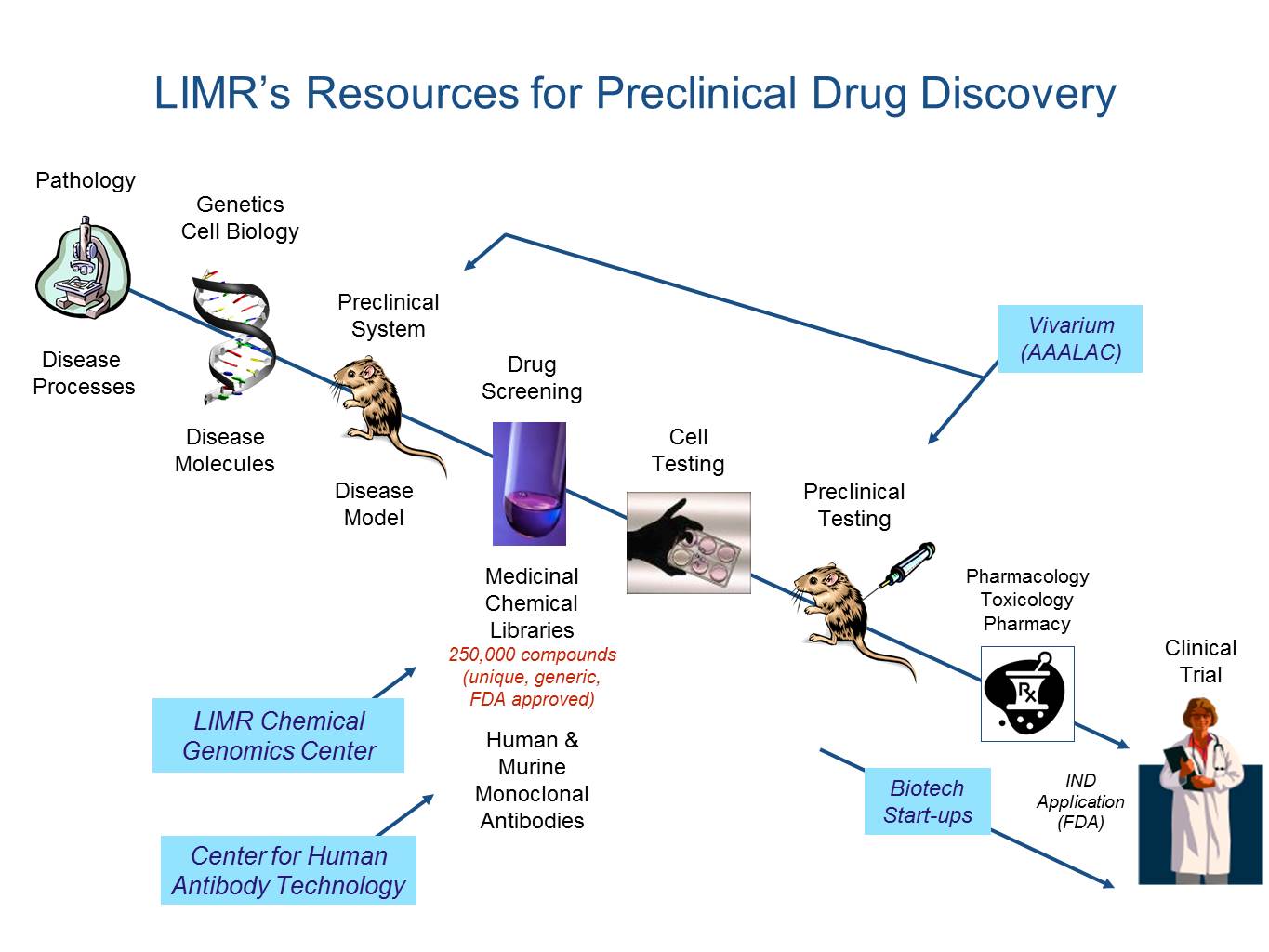
LIMR Chemical Genomics Center (LCGC)
LIMR Chemical Genomics Center (LCGC) has developed innovative technologies and approaches to catalyze medicinal discovery. We have developed a translational-research framework for public-private partnering called Double-Blinded Drug Discovery (DBD2)®. It aims to connect university and industrial scientists exploring the druggability of emerging medicinal targets.
RiboNova, Inc.
RiboNova, Inc. is developing small molecule drugs against a new family of targets: defective transfer RNAs. Its focus is on therapies that will be used in the treatment of cancer. CEO is Nigel Webb, and CSO is Tony Ford-Hutchinson, former head of Merck basic research.
Center for Human Antibody Technology (CHAT)
Center for Human Antibody Technology (CHAT) uses a human antibody cloning platform technology developed by LIMR's Scott Dessain, MD, PhD. CHAT uses this platform along with a state-of-the-art, high throughput antibody production and screening facility, to produce human antibodies for use in the treatment of infectious disease, cancer and neurological illnesses.
For more information, contact Scott Dessain, MD, PhD, at 484.476.6516 or [email protected].
